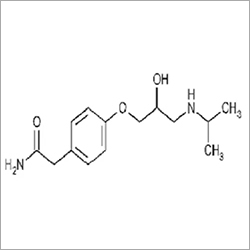
Atenolol pharmaceutical raw material
Product Details:
- Color White to off-white
- Storage Store in a cool, dry place; protect from light and moisture
- Molecular Formula C14H22N2O3
- Loss on Drying 0.5%
- Solubility Freely soluble in water, sparingly soluble in methanol, practically insoluble in chloroform and ether
- HS Code 29221990
- Taste Bitter
- Click to View more
X
Atenolol pharmaceutical raw material Price And Quantity
- INR
- 1 kg Kilograms
Atenolol pharmaceutical raw material Product Specifications
- C14H22N2O3
- >99%
- Pharmaceutical Raw Material
- Solid
- 0.5%
- White to off-white
- Store in a cool, dry place; protect from light and moisture
- Pharmaceutical Grade
- 249-020-3
- 4-(2-Hydroxy-3-isopropylaminopropoxy)phenylacetamide
- Non-poisonous in the raw phase, but for pharmaceutical use only
- 0.001%
- C14H22N2O3
- White to off-white crystalline powder
- 4.0 - 5.5 (1% solution)
- 36 months
- Typically 90% < 75 microns
- Not available (decomposes)
- Used in the manufacturing of antihypertensive drugs; beta-blocker for management of cardiovascular diseases
- 29221990
- 266.34 g/mol
- Bitter
- Freely soluble in water, sparingly soluble in methanol, practically insoluble in chloroform and ether
- 152-155 C
- Odorless
- 29122-68-7
- Atenolol
Atenolol pharmaceutical raw material Trade Information
- mumbai
- 100 Kilograms Per Day
- 1 Days
- Contact us for information regarding our sample policy
- All India
Product Description
CAS number: 29122-68-7
Chemical Formula: C14H22N2O3
For the management of hypertention and long-term management of patients with angina pectoris
Atenolol, a competitive beta(1)-selective adrenergic antagonist, has the lowest lipid solubility of this drug class. Although it is similar to metoprolol, atenolol differs from pindolol and propranolol in that it does not have intrinsic sympathomimetic properties or membrane-stabilizing activity. Atenolol is used alone or with chlorthalidone in the management of hypertension and edema.
Exceptional Pharmaceutical Quality
Atenolol meets stringent quality specifications, including high purity (>99%), minimal residue on ignition (0.1%), and ultra-low heavy metal content (0.001%). It passes identification tests (IR, HPLC) and offers reliable assay results, ensuring suitability for pharmaceutical production and regulatory compliance.
Safety and Stability Assured
Designed for optimal stability, Atenolol remains effective for up to 36 months when stored under recommended conditions. It is non-poisonous in raw form and passes all USP/BP/EP microbial and impurity standards. This ensures pharmaceutical manufacturers receive a safe, consistent material for their formulation needs.
Versatile and Efficient Packaging
Atenolol raw material is packaged in robust 25 kg fiber drums, tailored for bulk transport and storage. Customized packaging options are also available to meet specific client requirements, guaranteeing protection from moisture and light throughout the products shelf life.
FAQs of Atenolol pharmaceutical raw material:
Q: How is atenolol pharmaceutical raw material used in drug manufacturing?
A: Atenolol is primarily used in the formulation of antihypertensive drugs as a beta-blocker. Its raw powder form is processed further by pharmaceutical companies to produce prescription medications for managing cardiovascular conditions such as hypertension and angina.Q: What quality standards does atenolol raw material conform to?
A: This raw material conforms to USP, BP, and EP compendial standards. It meets strict requirements for purity, identification, microbial limits, related substances, and physical properties, ensuring quality and safety for pharmaceutical use.Q: When should atenolol be stored and handled to maintain stability?
A: Atenolol should always be stored in a cool, dry place away from direct light and moisture. Under these recommended conditions, the raw material remains stable and effective for up to 36 months from the manufacturing date.Q: Where can atenolol pharmaceutical raw material be sourced?
A: Atenolol is available from accredited distributors, exporters, manufacturers, suppliers, and traders in India. It can be ordered in bulk quantities, typically shipped in fiber drums, or customized packaging as required by the buyer.Q: What is the process for confirming the quality of atenolol before use?
A: Quality confirmation involves identification tests using IR and HPLC, checking assay (on dried basis), microbial limits, impurity analysis, specific optical rotation, and assessment of physical characteristics including particle size and pH. Each batch must conform to the specified standards before pharmaceutical application.Q: Which benefits does atenolol raw material offer to pharmaceutical manufacturers?
A: Atenolol provides high reliability, regulatory compliance, excellent solubility in water, and safe handling due to its non-toxic nature in raw form. Its consistent purity and stability simplify manufacturing processes, ensuring uniformity and efficacy in finished products.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email






 Call Me Free
Call Me Free
