Welcome to Our Company
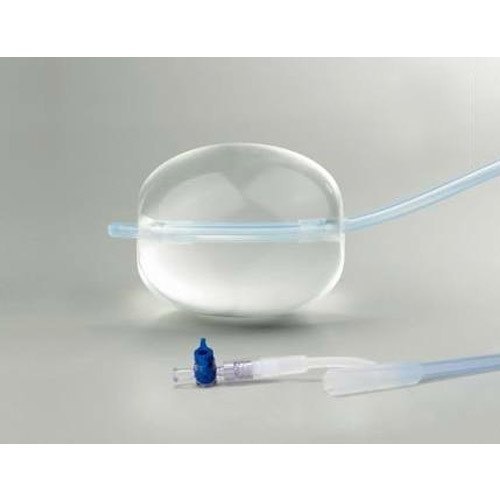
Bakri Balloon
Product Details:
- Particle Size Not applicable (solid device)
- Processing Type Sterile, single-use
- Advantage Rapid, minimally invasive control of uterine bleeding
- Solubility Not soluble in water
- Density Gram per cubic centimeter(g/cm3)
- Application Intrauterine balloon tamponade for obstetric bleeding
- Purity(%) Medical Grade (99%+) purity materials
- Click to View more
X
Bakri Balloon Price And Quantity
- 1 Unit
Bakri Balloon Product Specifications
- Medical grade silicone (Balloon), PVC (catheter)
- Gram per cubic centimeter(g/cm3)
- <1%
- Medical Grade (99%+) purity materials
- Intrauterine balloon tamponade for obstetric bleeding
- 500 mL maximum inflation volume, catheter length 54 cm
- Rapid, minimally invasive control of uterine bleeding
- Insert intrauterine, inflate with sterile fluid, monitor patient per hospital protocol
- Not soluble in water
- Control of postpartum hemorrhage (PPH) in obstetric care
- Store in a cool, dry place, away from direct sunlight
- Women experiencing postpartum hemorrhage
- Sterile, single-use
- Inflatable Balloon Catheter
- Bakri Balloon
- Surgical Grade
- Not applicable (solid device)
- 3 years
- Gynecology Medical Device
Bakri Balloon Trade Information
- mumbai
- Telegraphic Transfer (T/T)
- 1 Week
- Yes
- Contact us for information regarding our sample policy
- All India
Product Description
The Bakri Postpartum Balloon with Rapid Instillation Components is used to provide temporary control or reduction of postpartum uterine bleeding when conservative management is warranted.Purpose-Built for Postpartum Hemorrhage Control
The Bakri Balloon serves as an intrauterine tamponade, swiftly addressing severe uterine bleeding following childbirth. Its design prioritizes patient safety, offering easy insertion, controlled inflation with sterile saline, and prompt hemostasis, helping reduce complications and the need for surgical intervention.
Exceptional Materials and Safety Standards
Fabricated from 99%+ pure, medical-grade silicone with a PVC catheter, the Bakri Balloon ensures both flexibility and robust performance. Gamma-ray sterilization and leak-resistant construction safeguard sterility and efficiency, while radiopaque markings enhance placement visibility during follow-up imaging.
Practical, User-Oriented Design
Each Bakri Balloon is individually packaged for sterile, single-use application. Detailed volume graduations, a 500 mL maximum capacity, and a 54 cm catheter length allow clinicians to adjust the device precisely according to each patients clinical scenario, promoting optimal outcomes in postpartum care.
FAQs of Bakri Balloon:
Q: How is the Bakri Balloon used to manage postpartum hemorrhage?
A: The Bakri Balloon is used intrauterinely to control postpartum hemorrhage by inserting it into the uterus after childbirth and inflating it with sterile saline. The balloon applies gentle, consistent pressure to the uterine walls, helping to stop excessive bleeding quickly and effectively.Q: What are the benefits of using the Bakri Balloon over other methods?
A: This device provides a rapid, minimally invasive alternative to surgical interventions for postpartum hemorrhage, enabling effective bleeding control while preserving future fertility. Its transparent, radiopaque design allows for visual confirmation and monitoring, contributing to better patient safety and outcomes.Q: When should the Bakri Balloon be considered for use?
A: The device is recommended for women who experience moderate to severe postpartum hemorrhage unresponsive to standard medical therapy. It should be inserted promptly once bleeding has been identified and other conservative methods have not achieved control.Q: Where is the Bakri Balloon typically used in a clinical setting?
A: The Bakri Balloon is primarily used in hospital obstetric units and delivery rooms by trained healthcare professionals. It is intended for immediate intrauterine application following the diagnosis of postpartum hemorrhage.Q: What process should clinicians follow when using the Bakri Balloon?
A: Clinicians should insert the sterile, deflated balloon into the uterus, inflate it with sterile saline using the integrated port up to the marked capacity, and monitor the patient as per hospital protocol. Placement and inflation should be confirmed visually and through imaging if required.Q: What safety features does the Bakri Balloon offer to patients?
A: Key safety features include a leak-resistant design to prevent fluid leakage, radiopaque construction for monitoring via imaging, individual sterile packaging, soft medical-grade silicone material, and volume markings to avoid overinflationall contributing to safer postpartum hemorrhage control.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email