Welcome to Our Company
Mebeverine raw material
Product Details:
- Molecular Weight 429.55 g/mol
- Boiling point Not easily available due to decomposition
- Loss on Drying 0.5% Max
- Particle Size Micronized (as per requirement)
- EINECS No 220-446-6
- Storage Store in a cool, dry, well-ventilated area away from incompatible substances
- Color White to off-white
- Click to View more
X
Mebeverine raw material Price And Quantity
- 1 Kilograms
- INR
Mebeverine raw material Product Specifications
- 0.5% Max
- Not easily available due to decomposition
- 429.55 g/mol
- Store in a cool, dry, well-ventilated area away from incompatible substances
- 220-446-6
- Micronized (as per requirement)
- 5 years
- White to off-white
- Active Pharmaceutical Ingredient (API)
- Solid
- Pharmaceutical Grade
- 29420090
- Bitter
- Practically insoluble in water, freely soluble in methylene chloride, slightly soluble in ethanol
- Neutral (5.5 - 7.5 in solution)
- C25H35NO5
- Odorless
- No (when used as directed in pharmaceuticals)
- Used in the manufacture of antispasmodic drugs, treatment of irritable bowel syndrome (IBS)
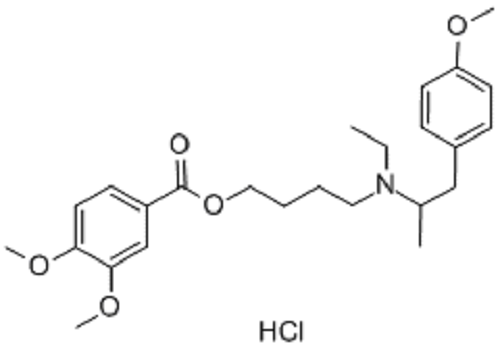
- Mebeverine
- 122-125C
- 3,4-Dimethoxybenzoic acid 4-(ethyl(2-methylphenyl)amino)butyl ester
- White to almost white crystalline powder
- 2753-45-9
- (Provided on request or see molecular diagram references)
- 0.001% Max
- 99% Min
Mebeverine raw material Trade Information
- 100 Kilograms Per Week
- 1 Week
- All India
Product Description
CAS number: 6/7/3625
Chemical Formula: C25H35NO5
Mebeverine is a drug used to alleviate some of the symptoms of irritable bowel syndrome. It works by relaxing the muscles in and around the gut
Superior Purity and Safety Profile
With a minimum assay of 99% and compliance to related substances (not more than 0.1%) and ICH Q3C residual solvent guidelines, Mebeverine ensures optimal safety and efficacy in pharmaceutical applications. Its heavy metal content is kept exceptionally low (0.001% max), supporting the highest standards for medicinal use.
Stable and Versatile for Pharmaceutical Manufacturing
Mebeverine is stable under recommended storage conditions and is supplied in secure packaging such as double PE bags in fiber drums, or customized as per client requirements. Its solid, micronized form enables versatile integration into drug formulations for reliable therapeutic outcomes.
Key Usage in Antispasmodic Drug Production
This API is vital in the manufacture of antispasmodic drugs, especially for the management and treatment of irritable bowel syndrome (IBS). The compounds bitter, odorless, and practically water-insoluble properties are well-suited for tablet and capsule preparations, resulting in targeted relief for affected patients.
FAQs of Mebeverine raw material:
Q: How is Mebeverine typically used in the pharmaceutical industry?
A: Mebeverine is primarily used as an active pharmaceutical ingredient in the manufacture of antispasmodic drugs designed to treat irritable bowel syndrome (IBS) and related gastrointestinal disorders.Q: What are the main quality and safety standards Mebeverine complies with?
A: This API meets pharmaceutical-grade standards with an assay of not less than 99%, residual solvent levels compliant with ICH Q3C, and related substances limited to not more than 0.1%. Heavy metal content is tightly controlled at a maximum of 0.001%.Q: When should Mebeverine be stored to maintain its stability and shelf life?
A: Mebeverine should be stored in a cool, dry, well-ventilated area away from incompatible substances. These conditions help preserve its stability and ensure a shelf life of up to 5 years.Q: Where is Mebeverine manufactured and distributed?
A: Mebeverine is produced and distributed in India by various entities, including manufacturers, suppliers, exporters, distributors, and traders, ensuring availability for global pharmaceutical needs.Q: What benefits does the high purity of Mebeverine offer in drug formulations?
A: The high purity of Mebeverine (99% min assay) ensures reliable therapeutic performance, minimal side effects, and consistent safety for patients suffering from IBS and other conditions requiring antispasmodic treatment.Q: How is Mebeverine packaged for export and industrial use?
A: Mebeverine is typically packed in double polyethylene bags placed inside fiber drums for maximum protection, or according to specific customer requirements, to maintain product integrity during transportation.Q: What is the process for confirming the identity and quality of Mebeverine?
A: The identity and quality are verified through IR/UV spectroscopic identification, and all quality parameters are reviewed to ensure compliance with pharmaceutical standards before shipment or use in drug formulations.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email






 Call Me Free
Call Me Free
