Welcome to Our Company
PHENYLEPHRINE HCL
Product Details:
- Solubility Freely soluble in water; sparingly soluble in ethanol
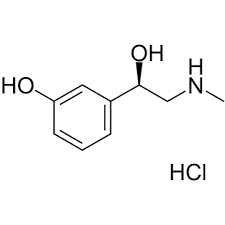
- Structural Formula C9H13NO2HCl
- Smell Odorless
- Melting Point 143-146C
- Shelf Life 5 Years
- Heavy Metal (%) 0.001%
- Ph Level 4.5 - 6.5 (1% aqueous solution)
- Click to View more
X
PHENYLEPHRINE HCL Product Specifications
- Used as a nasal decongestant, to treat hypotension, and in eye drops for pupil dilation
- As per specification (typically micronized)
- 2933
- NO
- C9H13NO2HCl
- White
- 98% Min
- 203.67 g/mol
- Characteristic
- Store in a cool, dry place, protected from light
- White to almost white crystalline powder
- 61-76-7
- Solid
- 0.5%
- 200-510-5
- 143-146C
- Odorless
- Freely soluble in water; sparingly soluble in ethanol
- C9H13NO2HCl
- 4.5 - 6.5 (1% aqueous solution)
- Phenylephrine HCl
- Active Pharmaceutical Ingredient (API)
- 5 Years
- 0.001%
- Pharmaceutical Grade
- Phenylephrine Hydrochloride
PHENYLEPHRINE HCL Trade Information
- mumbai
- Telegraphic Transfer (T/T)
- Yes
- Contact us for information regarding our sample policy
- Western Europe, Australia, Central America, Middle East, South America, Asia, Eastern Europe, North America, Africa
- All India
Product Description
Reliable Quality and Specifications
Phenylephrine HCl meets stringent pharmacopoeial quality requirements. With an assay range between 98.0% and 101.0% on a dried basis, related substances 0.5%, and heavy metals 0.001%, the product guarantees superior purity and stability. Its white crystalline appearance and micronized particle size optimize formulation compatibility for pharmaceutical manufacturers.
Versatile Pharmaceutical Applications
This API is ideal for use in nasal decongestants, ophthalmic solutions for pupil dilation, and treatments for hypotension. Its excellent solubility in water and stable pH range (4.5 - 6.5) make it suitable for a variety of liquid and solid dosage forms commonly required in modern healthcare.
Safe Handling and Packaging
Packaged in HDPE drums with double polyethylene bags, Phenylephrine HCl is protected from moisture and contamination. Being non-poisonous and compliant with all pharmacopeia standards, it is safe to transport, store, and handle, ensuring product integrity from manufacturer to end-user.
FAQs of PHENYLEPHRINE HCL:
Q: How is Phenylephrine HCl typically used in medical applications?
A: Phenylephrine HCl is primarily used as a nasal decongestant, in eye drops for pupil dilation, and to manage hypotension in various clinical settings. Its chemical stability and pharmacological properties make it essential for effective and targeted pharmaceutical formulations.Q: What are the main quality standards Phenylephrine HCl complies with?
A: Phenylephrine HCl meets all major pharmacopoeial requirements regarding identity, impurities, related substances (0.5%), heavy metals (0.001%), and microbial limits, ensuring it is safe for use in pharmaceutical manufacturing.Q: When should Phenylephrine HCl be used in pharmaceutical formulations?
A: This API is recommended whenever formulating nasal sprays, ophthalmic solutions, or medications aimed at managing hypotension. Its reliable assay (98.0% - 101.0%) and regulatory compliance make it suitable across a wide spectrum of medicines.Q: Where is Phenylephrine HCl manufactured and how is it packaged for quality assurance?
A: Manufactured in India, Phenylephrine HCl is distributed, exported, and supplied globally. It is securely packaged in HDPE drums lined with double polyethylene bags, which helps preserve quality and prevent contamination during storage and shipping.Q: What is the process for ensuring the purity and stability of Phenylephrine HCl?
A: Manufacturers rigorously test each batch for assay value, related substances, heavy metals, and microbial limits according to pharmacopoeial guidelines. Controlled storage in cool, dry conditions, away from light, further preserves its purity and extends its shelf life.Q: What benefits does Phenylephrine HCl offer to pharmaceutical manufacturers?
A: Its high purity (minimum 98%), excellent solubility, compliance with international standards, and stable shelf life make Phenylephrine HCl a highly reliable API. These qualities ensure consistent performance and safety in finished dosage forms for patients.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email







 Call Me Free
Call Me Free
