Welcome to Our Company
Ranitidine
Product Details:
- Heavy Metal (%) < 0.001%
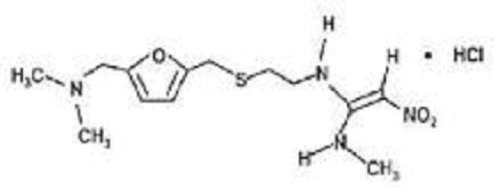
- Structural Formula Refer to image
- Color White or off-white
- EINECS No 266-583-1
- Poisonous Non-poisonous
- Molecular Weight 314.41 g/mol
- Solubility Freely soluble in water
- Click to View more
X
Ranitidine Price And Quantity
- 1 kg Kilograms
- INR
Ranitidine Product Specifications
- 266-583-1
- Non-poisonous
- Refer to image
- < 0.001%
- White or off-white
- 5 Years
- Not applicable (decomposes)
- Not more than 0.5%
- C13H22N4O3S
- 314.41 g/mol
- Freely soluble in water
- >99%
- Bitter
- Ranitidine
- White to pale yellow powder
- Not less than 5.2
- 141-142C
- Micronized (customizable)
- 66357-35-5
- Ranitidine Hydrochloride
- Odorless
- Pharmaceutical Grade
- Used for the treatment of peptic ulcer and gastric reflux
- Store in a cool, dry place
- 29420090
- Medicine Raw Materials
Ranitidine Trade Information
- mumbai
- 100 Kilograms Per Week
- 1 Week
- All India
Product Description
Grab Yours Ranitidine at a Remarked Reduced Price! This top-notch pharmaceutical raw material boasts a towering assay of 99.5% and exceeds USP/BP standards for identification and microbial limits. With impurity levels below 0.1% and moisture content under 0.5%, Ranitidine Hydrochloride is an ideal choice for pharmaceutical formulations, APIs, and more. Get It Now in a secure 25kg HDPE drumcustom packaging available upon request. Stable, non-allergenic, and with impressive monthly supply, Ranitidine from India is the smart choice for distributors, exporters, and manufacturers seeking excellence.
Key Features & Applications of Ranitidine
Ranitidine is a micronized, pharmaceutical-grade raw material widely utilized in hospital pharmacies, laboratories, and industrial settings. Its towering purity and stability make it top-notch for manufacturing anti-ulcer medications, especially for peptic ulcer and gastric reflux treatment. Odorless and non-toxic, its suitable for therapeutic formulations requiring a bitter, freely soluble powder. High material integrity ensures compliance with regulatory standards, offering reliable results wherever stringent quality is required.
Market Value & Packaging Details of Ranitidine
The asking price for Ranitidine represents remarkable market value, driven by its superior purity, stability, and packaging standards. Shipped goods depart from major Indian FOB ports, and prompt delivery is guaranteed with a seven-day lead time. Buyers can choose secure 25kg HDPE drum packaging or customize to suit specific needs. Flexible payment terms ensure smooth transactions for distributors, exporters, and pharmaceutical manufacturers, making procurement efficient and cost-effective.
Key Features & Applications of Ranitidine
Ranitidine is a micronized, pharmaceutical-grade raw material widely utilized in hospital pharmacies, laboratories, and industrial settings. Its towering purity and stability make it top-notch for manufacturing anti-ulcer medications, especially for peptic ulcer and gastric reflux treatment. Odorless and non-toxic, its suitable for therapeutic formulations requiring a bitter, freely soluble powder. High material integrity ensures compliance with regulatory standards, offering reliable results wherever stringent quality is required.
Market Value & Packaging Details of Ranitidine
The asking price for Ranitidine represents remarkable market value, driven by its superior purity, stability, and packaging standards. Shipped goods depart from major Indian FOB ports, and prompt delivery is guaranteed with a seven-day lead time. Buyers can choose secure 25kg HDPE drum packaging or customize to suit specific needs. Flexible payment terms ensure smooth transactions for distributors, exporters, and pharmaceutical manufacturers, making procurement efficient and cost-effective.
FAQs of Ranitidine:
Q: How is Ranitidine typically used in pharmaceutical applications?
A: Ranitidine is chiefly used in pharmaceutical formulations as an API for the treatment of peptic ulcers and gastric reflux, ensuring effective relief and compliance with global medical standards.Q: What are the material features that make Ranitidine suitable for pharmaceutical use?
A: Ranitidine boasts a high assay (99.5%), compliance with USP/BP standards, low impurity and moisture content, and is non-allergenic, stable, and available in a bitter, freely soluble powder form.Q: When can I expect delivery after placing an order?
A: You can expect shipped goods to be delivered promptly, with a lead time typically within seven days from order confirmation, each batch shipped under optimal transport conditions.Q: Where is Ranitidine exported from and what is its supply capability?
A: Ranitidine is exported from India and offers a robust monthly supply ability of up to 25,000 kg, ensuring reliability and bulk availability for global pharmaceutical distributors.Q: What are the recommended storage and transport conditions?
A: Ranitidine should be stored in a cool, dry place away from sunlight and humidity. During transport, it is kept under controlled conditions to maintain its stability and top-notch quality.Q: How is the packaging of Ranitidine managed for bulk shipments?
A: Bulk shipments typically come in secure 25kg HDPE drums, though packaging can be customized upon request to meet specific client needs and facilitate safe international transport.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email






 Call Me Free
Call Me Free
