Welcome to Our Company
tolvaptan
Product Details:
- Color White to off-white
- Melting Point 230-234C
- Molecular Weight 448.94 g/mol
- Particle Size 90% 10 microns
- Heavy Metal (%) 0.001%
- Molecular Formula C26H25ClN2O3
- Solubility Slightly soluble in water, soluble in DMSO and methanol
- Click to View more
X
tolvaptan Price And Quantity
- 1 Kilograms
tolvaptan Product Specifications
- 230-234C
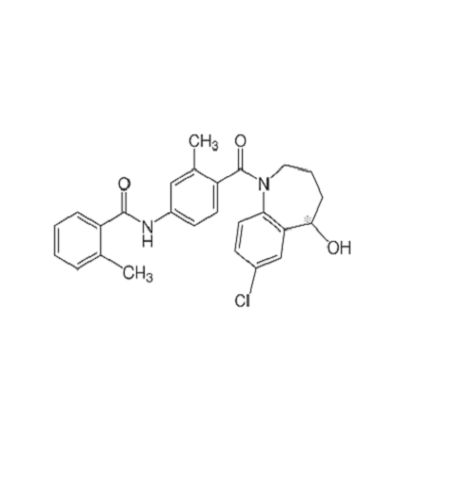
- 4-[(7-chloro-2,3,4,5-tetrahydro-5-hydroxy-1H-1-benzazepin-1-yl)carbonyl]-o-tolu-m-toluidide
- 90% 10 microns
- 448.94 g/mol
- Used in the treatment of hyponatremia associated with congestive heart failure, cirrhosis, and syndrome of inappropriate antidiuretic hormone (SIADH)
- 0.001%
- 29420090
- White to off-white crystalline powder
- Slightly soluble in water, soluble in DMSO and methanol
- 150683-30-0
- C26H25ClN2O3
- NO
- Store at 2-8C, in a dry and well-ventilated place
- Solid
- >=98%
- 0.5%
- Odorless
- See image
- Pharmaceutical Grade
- 5 Years if properly stored
- Tolvaptan
- White to off-white
- Active Pharmaceutical Ingredient (API)
tolvaptan Trade Information
- Telegraphic Transfer (T/T)
- 1 Week
- All India
Product Description
is an aquaretic drug that functions as a selective, competitive vasopressin receptor 2 (V2) antagonist used to treat hyponatremia (low blood sodium levels) associated with congestive heart failure, cirrhosis, and the syndrome of inappropriate antidiuretic hormone (SIADH).
Pharmaceutical-Grade Safety and Quality
Our Tolvaptan API is manufactured and tested to the highest pharmaceutical standards. Rigorous identification methods, including IR, HPLC, and Mass Spectrometry, ensure product integrity. Each batch meets strict impurity, related substances, and residual solvent specifications as per the latest pharmacopeial guidelines, providing clients with dependable quality in every order.
Reliable Packaging and Storage
Tolvaptan is protected with double polyethylene bags inside HDPE drums to preserve product stability during storage and shipment. With a recommended storage temperature of 2-8C and dry, ventilated conditions, the product maintains its chemical integrity for up to five years, assuring ease of logistics for global distribution partners.
FAQs of tolvaptan:
Q: How is Tolvaptan packaged for shipment and storage?
A: Tolvaptan is securely packed in double polyethylene bags which are then placed inside high-density polyethylene (HDPE) drums. This packaging ensures product stability, prevents contamination, and facilitates safe transportation.Q: What are the identification methods used for Tolvaptan quality assurance?
A: Each batch of Tolvaptan is identified and verified using IR spectroscopy, HPLC, and Mass Spectrometry, guaranteeing its authenticity and compliance with pharmacopeial standards.Q: When should Tolvaptan be used, and for what therapeutic indications?
A: Tolvaptan is used in the formulation of pharmaceutical products intended for the treatment of hyponatremia associated with congestive heart failure, cirrhosis, and SIADH, as directed by a qualified healthcare provider.Q: Where should Tolvaptan be stored to maintain its shelf life and quality?
A: Store Tolvaptan at a temperature between 2-8C in a dry, well-ventilated area and protect it from direct sunlight to ensure optimal shelf life and quality retention for up to five years.Q: What process ensures Tolvaptans impurities are within acceptable levels?
A: Tolvaptan undergoes stringent quality testing, including analyses for impurities and related substances as specified by pharmacopeial standards and residual solvent content within ICH limits, to ensure product safety and efficacy.Q: How is Tolvaptan beneficial for pharmaceutical manufacturers?
A: Pharmaceutical manufacturers benefit from Tolvaptans high purity (98%), consistent particle size, reliable supply in ready stock, and compliance with all international pharmacopeial and regulatory norms, supporting efficient and quality production.Q: What is the typical appearance and physical form of Tolvaptan?
A: Tolvaptan is supplied as a white to off-white crystalline powder, appearing odorless and solida form ideal for pharmaceutical formulation processes.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email