Anastrozole
Product Details:
- Heavy Metal (%) Not more than 0.001%
- Poisonous NO
- Molecular Formula C17H19N5
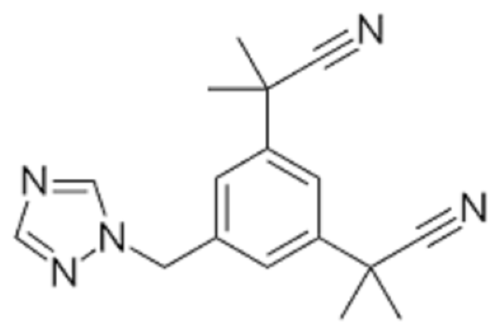
- Structural Formula C17H19N5
- Loss on Drying Not more than 0.5%
- Storage Store in a cool, dry place away from light
- Color White or almost white
- Click to View more
Anastrozole Price And Quantity
- 1 kg Kilograms
- INR
Anastrozole Product Specifications
- Pharmaceutical Grade
- Not more than 0.001%
- NO
- Store in a cool, dry place away from light
- Solid
- White to off-white crystalline powder
- C17H19N5
- Anastrozole
- Active Pharmaceutical Ingredient (API)
- Odorless
- 99% minimum
- White or almost white
- 2,2-(5-(1H-1,2,4-Triazol-1-ylmethyl)-1,3-phenylene)di(2-methylpropanenitrile)
- C17H19N5
- 293.37 g/mol
- Not more than 0.5%
- 81-82C
- 2 years
- 120511-73-1
- Used in the treatment of breast cancer
- 2933.99.90
- Slightly soluble in water, soluble in ethanol, methanol, and acetone
Anastrozole Trade Information
- mumbai
- 100 Kilograms Per Day
- 1 Week
- All India
Product Description
CAS number: 120511-73-1
Chemical Formula: C17H19N5
Anastrozole is a drug indicated in the treatment of breast cancer in post-menopausal women. It is used both in adjuvant therapy (i.e. following surgery) and in metastatic breast cancer. It decreases the amount of estrogens that the body makes. Anastrozole belongs in the class of drugs known as aromatase inhibitors. It inhibits the enzyme aromatase, which is responsible for converting androgens (produced by women in the adrenal glands) to estrogens. For adjuvant treatment of hormone receptor positive breast cancer , as well as hormonal treatment of advanced breast cancer in post-menopausal women. Has also been used to treat pubertal gynecomastia and McCune-Albright syndrome; however, manufacturer states that efficacy for these indications have not been established.
Pharmaceutical Standards and Safety
Anastrozole meets rigorous pharmacopeial and ICH guidelines, ensuring its safety and efficacy for medicinal applications. It is tested for purity, single impurities, heavy metals, and microbial limits, guaranteeing a high-quality API suitable for pharmaceutical formulations. With loss on drying not surpassing 0.5% and heavy metals maintained below 0.001%, the product stands out for its reliable composition and safety profile.
Physical and Chemical Properties
This API appears as a white or almost white crystalline solid and offers optimal solubility in ethanol, methanol, and acetone, though it is only slightly soluble in water. With a molecular formula of C17H19N5 and weight of 293.37 g/mol, Anastrozoles chemical identity is confirmed by infrared spectroscopy (IR) analyses, complying with reference standards at every stage. The melting point is consistent at 81-82C, and the compound remains odorless during handling.
Optimal Storage and Handling
To preserve Anastrozoles quality and stability, it is packaged in securely sealed plastic drums, safeguarding from moisture and light. Recommended storage conditions include keeping the API in a cool, dry environment, away from direct sunlight. When stored as instructed, the shelf life extends up to two years, maintaining all pharmacological and physical properties essential for manufacturing and distribution.
FAQs of Anastrozole:
Q: How should Anastrozole be stored to maintain its effectiveness?
A: Anastrozole should be stored in a cool, dry place away from direct light, inside sealed plastic drums or appropriate containers. This preserves its purity and ensures a shelf life of up to two years.Q: What processes are followed to confirm the identity and purity of Anastrozole?
A: Identity is confirmed by infrared (IR) analysis, and purity is ensured through compliance with reference standards and rigorous pharmacopeial assays, where purity ranges from 98% to 102% and impurities are strictly limited to not more than 0.1%.Q: When is Anastrozole commonly used in medical treatment?
A: Anastrozole is primarily used in the treatment of breast cancer, acting as a pharmaceutical ingredient in formulations prescribed by healthcare professionals.Q: Where is Anastrozole manufactured and supplied from?
A: Anastrozole is manufactured, supplied, and exported from India by distributors, exporters, manufacturers, suppliers, and traders specializing in pharmaceutical-grade APIs.Q: What are the benefits of using pharmaceutical-grade Anastrozole?
A: Pharmaceutical-grade Anastrozole offers high purity (at least 99%), strict impurity controls, and compliance with safety standards, ensuring efficacy and minimized risk for patients.Q: How does the packing of Anastrozole aid in product safety?
A: The product is packed in sealed plastic drums or as required to prevent contamination, moisture ingress, and exposure to light, thereby safeguarding overall product quality during storage and transport.
Price:
- 50
- 100
- 200
- 250
- 500
- 1000+






 Call Me Free
Call Me Free
