Welcome to Our Company
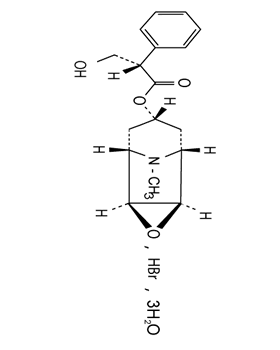
HYOSCINE HYDROBROMIDE IP
Product Details:
- Melting Point 202-204C
- EINECS No 204-756-4
- Structural Formula C17H21NO4HBr
- Ph Level 4.5 5.5 (1% solution)
- Storage Store in a cool, dry place, protected from light
- Smell Odorless
- HS Code 29420090
- Click to View more
X
HYOSCINE HYDROBROMIDE IP Product Specifications
- 204-756-4
- 202-204C
- 99.0%
- Active Pharmaceutical Ingredient (API)
- 4.5 5.5 (1% solution)
- 125-72-8
- Odorless
- Store in a cool, dry place, protected from light
- C17H21NO4HBr
- Micronized
- 29420090
- Scopolamine Hydrobromide
- IP (Indian Pharmacopoeia)
- White to off-white
- 384.26 g/mol
- C17H21NO4HBr
- 0.001%
- Solid
- 36 Months
- White to off-white crystalline powder
- Freely soluble in water; slightly soluble in ethanol
- 0.5%
- YES
- Pharmaceuticals, Antispasmodic, Antiemetic
- Bitter
- Hyoscine Hydrobromide IP
HYOSCINE HYDROBROMIDE IP Trade Information
- Telegraphic Transfer (T/T)
- 1 Per Day
- 1 Week
- Yes
- Australia, Central America, North America, South America, Eastern Europe, Western Europe, Middle East, Africa
- All India
Product Description
HYOSCINE HYDROBROMIDE IP stands as a best-selling, astounding Active Pharmaceutical Ingredient (API), celebrated by industry virtuosos for its champion performance in pharmaceutical applications. This customizable product features a purity 99.0%, with a confirmed optical rotation of +21 to +24, matching the strictest IP standards. Packaged in sealed HDPE drums or as required, it remains stable under recommended storage. A white to off-white crystalline powder, this antispasmodic and antiemetic API is freely soluble in water with a shelf life of 36 months. Buy from trusted manufacturers, exporters, and distributors in India.
Advanced API for Pharmaceutical Excellence
HYOSCINE HYDROBROMIDE IP is a distinguished Active Pharmaceutical Ingredient widely used as an antispasmodic and antiemetic in the pharmaceutical sector. Its application extends to the formulation of medicines addressing motion sickness and gastrointestinal disorders. Extra features such as exceptional purity, standardized micronized particle size, and compliance with IP regulations make it highly reliable for medicinal experts aiming to ensure optimal patient outcomes.
Strategic Markets and Secure Packaging Solutions
The main domestic market for HYOSCINE HYDROBROMIDE IP covers a broad spectrum of pharmaceutical exchanges across India, with strong handover protocols for product safety. Packaging details include robust, sealed HDPE drums ensuring sample integrity and value preservation during transit and storage. Internationally, this API is highly valued and exported globally, securing a champion position in global valuations for high-quality pharmaceutical raw materials.
Advanced API for Pharmaceutical Excellence
HYOSCINE HYDROBROMIDE IP is a distinguished Active Pharmaceutical Ingredient widely used as an antispasmodic and antiemetic in the pharmaceutical sector. Its application extends to the formulation of medicines addressing motion sickness and gastrointestinal disorders. Extra features such as exceptional purity, standardized micronized particle size, and compliance with IP regulations make it highly reliable for medicinal experts aiming to ensure optimal patient outcomes.
Strategic Markets and Secure Packaging Solutions
The main domestic market for HYOSCINE HYDROBROMIDE IP covers a broad spectrum of pharmaceutical exchanges across India, with strong handover protocols for product safety. Packaging details include robust, sealed HDPE drums ensuring sample integrity and value preservation during transit and storage. Internationally, this API is highly valued and exported globally, securing a champion position in global valuations for high-quality pharmaceutical raw materials.
FAQs of HYOSCINE HYDROBROMIDE IP:
Q: How is HYOSCINE HYDROBROMIDE IP primarily used in pharmaceuticals?
A: HYOSCINE HYDROBROMIDE IP serves as an antispasmodic and antiemetic, commonly found in medications for motion sickness, gastrointestinal disorders, and other related conditions.Q: What measures ensure the quality and safety of HYOSCINE HYDROBROMIDE IP during storage and shipping?
A: The API is packaged in sealed HDPE drums or as required, and it is stable under recommended conditionscool, dry, and protected from lightto maintain safety and integrity until handover.Q: Where can buyers source HYOSCINE HYDROBROMIDE IP from reliable suppliers?
A: Buyers can exchange directly with reputable manufacturers, distributors, and exporters in India, ensuring product authenticity and quality valuation.Q: What are the key benefits for pharmaceutical firms using this product?
A: The standout benefits include compliance with IP standards, high assay results, exceptional purity, micronized particle sizing, and a stable shelf life of 36 months, making it a consistent component for pharmaceutical formulations.Q: What packaging options are available for HYOSCINE HYDROBROMIDE IP?
A: The API is typically offered in sealed HDPE drums, but customizable packaging solutions are available to fit specific client requirements.Q: How is product quality validated before it reaches end-users?
A: Every batch undergoes identification, assay, microbial limit, and residue tests in line with Indian Pharmacopoeia regulations, ensuring a champion-grade product valuation.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email






 Call Me Free
Call Me Free
